News and Content
The Latest News from Agilex Biolabs
Author By Agilex BiolabsPosted on
Agilex Biolabs expands with a new Flow Cytometry Laboratory at QIMR Berghofer, boosting speed and precision in clinical research.
Author By Agilex BiolabsPosted on
Contracted by the South Australian Government, Agilex Biolabs is deploying world-class science to sa...
Author By Agilex BiolabsPosted on
Introduction – A New Chapter in Drug Development The use of biomarkers in medicine is not new....
Author By Agilex BiolabsPosted on
By Dr Hayley Kain, Pharmacokinetic Scientist at Agilex Biolabs Rapid Data. Smarter Decisions. No...
Author By Agilex BiolabsPosted on
By Dr. Kurt Sales, CSO Summary The following article focuses on the use of flow cytometry in c...
Author By Agilex BiolabsPosted on
By William Grenfell, Head of IT In today's digital age, information security is paramount. At Agi...
Author By Agilex BiolabsPosted on
By Kim Salvatore, Head of Client and Operational Services The bioanalytical and drug development...
Author By Agilex BiolabsPosted on
Research shows that Biotechnology companies have been facing significant challenges – with continu...
Author By Agilex BiolabsPosted on
By Dr. Kurt Sales, CSO Peripheral Blood Mononuclear Cells (PBMC) are critical for the pharmacodyn...
Author By Agilex BiolabsPosted on
For 3 decades, Agilex has supported biotech companies with CNS-targeted medicines. For almost 3 d...
Author By Agilex BiolabsPosted on
We took some time to sit down and talk with Jason so you could get to know him a bit better... My...
Author By Agilex BiolabsPosted on
We took some time to sit down and talk with Cameron so you could get to know him a bit better......
Author By Agilex BiolabsPosted on
We took some time to sit down and talk with Dr Sales so you could get to know him a bit better......
Author By Agilex BiolabsPosted on
Agilex is pleased to introduce Mallory Jolly as our new Senior Business Development Director to the...
Author By Agilex BiolabsPosted on
Agilex Demystifies the Rapidly Evolving Landscape of Drug Development Post-Covid, the field of bi...
Author By Agilex BiolabsPosted on
As Agilex Biolabs continues its global expansion and corporate evolution as part of the Healius network, Stephen McIntyre has been selected to take the position of Chief Executive Officer.
Author By Agilex BiolabsPosted on
As Agilex Biolabs continues its global expansion and corporate evolution as part of the Healius network, Stephen McIntyre has been selected to take the position of Chief Executive Officer.
Author By Agilex BiolabsPosted on
by Holly Stefl, Chief Commercial Officer It is with much enthusiasm that Agilex Biolabs introduce...
Author By Agilex BiolabsPosted on
by Holly Stefl, Chief Commercial Officer Agilex Biolabs is proud to introduce the West Coast of t...
Author By Agilex BiolabsPosted on
Agilex Biolabs, the leading bioanalytical laboratory supporting Australian clinical trials, has established its first satellite processing unit staffed with highly specialised technicians on the fifth floor of the CMAX Clinical Research facility in Adelaide, SA.
Author By Agilex BiolabsPosted on
As Agilex Biolabs continues its global expansion and corporate evolution as part of the Healius network, Stephen McIntyre has been selected to take the position of Chief Executive Officer.
Author By Agilex BiolabsPosted on
Rescue studies are a particular point of strength at Agilex Biolabs, where the bioanalytical experts are driven to solve challenges presented by what we call “the weird and wonderful”.
Author By Agilex BiolabsPosted on
On November 21, 2022, Agilex Biolabs was named this year’s winner for the Editor’s Choice Award for “Top company in clinical research-based development” in BioSpectrum Asia Excellence Awards.
Author By Agilex BiolabsPosted on
Agilex Biolabs, the leading bioanalytical laboratory supporting Australian clinical trials, has established its first satellite processing unit staffed with highly specialised technicians on the fifth floor of the CMAX Clinical Research facility in Adelaide, SA.
Author By Agilex BiolabsPosted on
Agilex Biolabs has expanded its capacity to better serve the biotech, pharmaceutical and animal health industry with the opening of its new in-vivo testing facility.
Author By Agilex BiolabsPosted on
Circulating tumor DNA (ctDNA) is tumor-deriver fragmented DNA which originates either directly from the tumor or from circulating tumor cells.
Author By Agilex BiolabsPosted on
Immunogenicity assessment of biotherapeutics is a regulatory requirement and key parameter for assessing the safety and efficacy of a drug candidate.
Author By Agilex BiolabsPosted on
Immunogenicity assessment is a critical safety attribute for biotherapeutics undergoing clinical development.
Author By Agilex BiolabsPosted on
Dr. Kurt Sales Appointed as Chief Scientific Officer at Agilex Biolabs Kurt Sales as Chief Scient...
Author By Agilex BiolabsPosted on
Learn how to improve Bioanalytical clinical outcomes by performing MetID characterisation. Leading...
Author By Agilex BiolabsPosted on
Large Molecule Facility Doubles Size of Australia’s Top Bioanalytical Laboratory Agilex Biolabs...
Author By Agilex BiolabsPosted on
Agilex Biolabs provides world-class toxicology services via its purpose-built GLP compliant facility...
Author By Agilex BiolabsPosted on
This year, we at Agilex Biolabs celebrate our 25th year supporting Biotechs’ d...
Author By Agilex BiolabsPosted on
Agilex Biolabs Pty Ltd has been purchased by one of Australia’s leading healthcare companies, Healius Limited
Author By Agilex BiolabsPosted on
With increasingly novel drug development programs, meaningful collection of subject samples has never been more relevant. Don’t let potential analyte instability mislead your program as it gets underway, causing costly time delays
Author By Agilex BiolabsPosted on
Agilex Biolabs Congratulates VGI Health Technology Limited for successful filing of a new PCT Patent Application on Transmucosal Delivery of Tocotrienols
Author By Agilex BiolabsPosted on
With the integration of leading toxicology company TetraQ into Agilex Biolabs’ service offering, A...
Author By Agilex BiolabsPosted on
Meet the Scientists series - Meer Dr Jason Geue, R& D Manager at Agilex Biolabs.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s most experienced and technologically advanced bioanalytical laboratory and the only biolab in the region with a dedicated toxicology unit, has been selected as a finalist for the Most Successful Early Phase Research (Preclinical & Phase I) category in the Citeline Awards 2021.
Author By Agilex BiolabsPosted on
https://youtu.be/dnyLFRPLEJo...
Author By Agilex BiolabsPosted on
Agilex Biolabs, the Australian specialist bioanalytical and toxicology laboratory facilities for clinical trials is partnering with Endpoints News to share the latest on “Non-clinical and clinical pathways for rapid vaccine development in Australia”, in a webinar hosted by Endpoints News Editor Arsalan Arif.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest and most technologically advanced regulated bioanalytical laboratory for clinical trials is showcasing its new $1.5m vaccine and immunobiology laboratory at BIO Asia Taiwan 2021. The facility, the most sophisticated in APAC, is designed for biotechs and pharma from around the world for advanced clinical research including RNA vaccines, siRNA/miRNA clinical targets and gene therapy studies.
Over the past 2 years, Agilex Biolabs has invested more than $3.5m in technology and systems at the APAC headquarters in Adelaide.
Over the past 2 years, Agilex Biolabs has invested more than $3.5m in technology and systems at the APAC headquarters in Adelaide.
Author By Agilex BiolabsPosted on
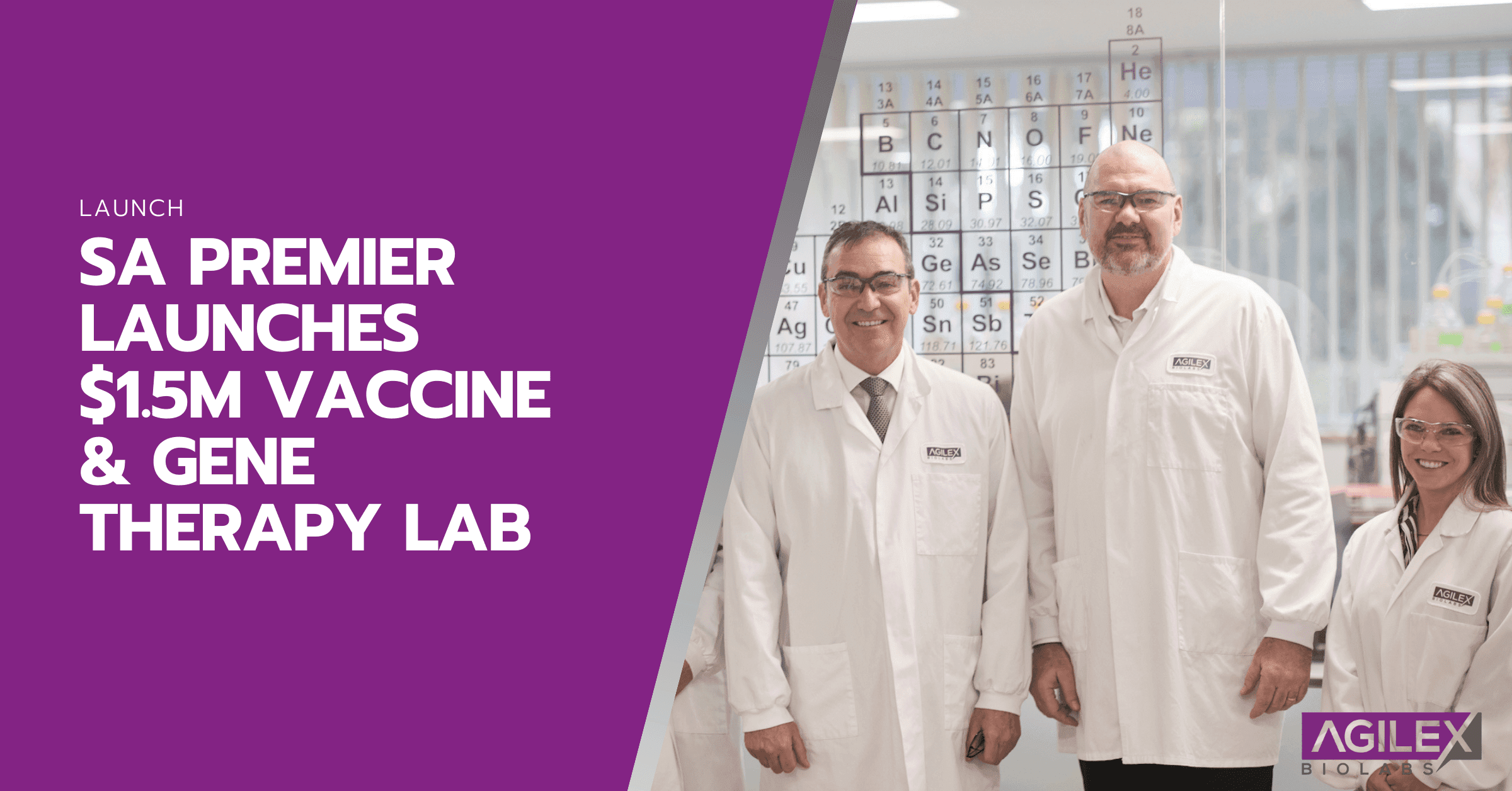
Agilex Biolabs, Australia’s largest and most technologically advanced regulated bioanalytical laboratory for clinical trials today announced that the Premier of South Australia the Hon Steven Marshall MP has launched its new $1.5m vaccine and immuno-biology laboratory. The facility, the most sophisticated in APAC, will attract biotechs and pharma from around the world for advanced clinical research.
Over the past 2 years, Agilex Biolabs has invested more than $3.5m in technology and systems at the APAC headquarters in Adelaide.
Over the past 2 years, Agilex Biolabs has invested more than $3.5m in technology and systems at the APAC headquarters in Adelaide.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest and most technologically advanced specialist bioanalytical laboratory for clinical trials announced for BIO Korea 2021 that their upgraded and expanded biolabs at the Australian headquarters is now complete.
The labs have been expanded by 30% to cater for increased demand for bioanalysis and biomarkers in clinical studies from clinical stage biotechs.
The labs have been expanded by 30% to cater for increased demand for bioanalysis and biomarkers in clinical studies from clinical stage biotechs.
Author By Agilex BiolabsPosted on
Inflammation is a complex biological process involving a host of resident and recruited immune cells which are mobilized to infiltrate tissues in response to invading pathogens. These immune cells boast elevated numbers and heightened activation as they churn out inflammatory mediators, which act in unison to coordinate and modulate the immune response.
Choosing the right bioanalytical platform for your immunology clinical trial is critical to data collection. But navigating the intricate inflammation process can be difficult. What do these platforms look like in use, and how should you go about selecting the right one?
Choosing the right bioanalytical platform for your immunology clinical trial is critical to data collection. But navigating the intricate inflammation process can be difficult. What do these platforms look like in use, and how should you go about selecting the right one?
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest and most technologically advanced specialist bioanalytical laboratory for clinical trials is partnering with Endpoints News to share the latest on deconvoluting inflammation and immunology for clinical trials, in a webinar hosted by Endpoints News Editor Arsalan Arif.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest and most technologically advanced specialist bioanalytical laboratory for clinical trials, said biotechs with first-in-human studies can leverage significant quality, speed and cost advantages working with their company TetraQ toxicology for pre-clinical research in Australia.
TetraQ is a NATA-accredited, GLP-recognised rodent toxicology facility.
TetraQ is a NATA-accredited, GLP-recognised rodent toxicology facility.
Author By Agilex BiolabsPosted on
This webinar examines the hot topics in immunoassay bioanalysis for biotechs navigating the immunogenicity and biomarker requirements for clinical trials. Join the discussion with panelists from Agliex Biolabs, Australia’s largest and most technologically advanced specialist bioanalytical laboratory for clinical trials, and B2S Life Sciences, a biotherapeutic enablement company advancing improved analytical methods and outcomes for developers of biotherapeutic drugs and diagnostics
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest and most technologically advanced specialist bioanalytical laboratory for clinical trials, today announced the purchase of leading biolab TetraQ, based out of the University of Queensland.
Agilex Biolabs CEO Jason Valentine said the Board and senior management at Agilex Biolabs were extremely pleased with the purchase of such a high-calibre bioanalytics company.
“TetraQ complements the Agilex Biolabs work in Australia and APAC with its expert staff and years of experience in regulated bioanalytical services,” he said.
Agilex Biolabs CEO Jason Valentine said the Board and senior management at Agilex Biolabs were extremely pleased with the purchase of such a high-calibre bioanalytics company.
“TetraQ complements the Agilex Biolabs work in Australia and APAC with its expert staff and years of experience in regulated bioanalytical services,” he said.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest and most technologically advanced specialist bioanalytical laboratory for clinical trials, today announced it will showcase its world-class immunoassay and immunobiology services for regulated bioanalysis at Bio-Europe 2021.
Author By Agilex BiolabsPosted on
Demand for APAC clinical trials is still on the rise as COVID numbers continue to fall across the region attracting biotechs wanting to restart stalled or paused clinical trials from around the globe. Indeed Australia recorded zero new cases in the past 25 days.
Author By Agilex BiolabsPosted on
Are you a highly experienced Study Director with proven experience in developing and validating flow cytometry assays (immunophenotyping and receptor occupancy assays) and/or cell-based neutralising or mode of action assays in a regulatory environment?
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s most advanced FDA-inspected specialist bioanalytical laboratory for clinical trials, today announced the roll-out of the GALEXI™ client portal for real-time study management.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s most advanced FDA-inspected specialist bioanalytical laboratory for clinical trials, today announced the roll-out of the GALEXI™ client portal for real-time study management.
Author By Agilex BiolabsPosted on
Agilex Biolabs is the leading bioanalytical laboratory in Asia-Pacific with the most advanced platforms for sensitive technologies. They include Sciex API 6500+ LC-MS/MS, Sciex API 4000 LC-MS/MS systems; Gyrolab™ Xplore, Luminex MAGPIX xPONENT ™, MSD Quickplex 120 and BD FACSymphony™ A3 20 colour flow cytometry analysers, so we have the capability to deliver any sized project.
Author By Agilex BiolabsPosted on
Register here (free)...
Author By Agilex BiolabsPosted on
Agilex Biolabs, the leading APAC biolabs for biotech clinical trials, announced today the launch of a Client Portal named GALEXI™.
The Agilex Biolabs technology team, with its Microsoft partner, accelerated the launch of GALEXI™ to deliver secure and enhanced communications during continued COVID challenges globally.
The Agilex Biolabs technology team, with its Microsoft partner, accelerated the launch of GALEXI™ to deliver secure and enhanced communications during continued COVID challenges globally.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s most advanced FDA-inspected specialist bioanalytical laboratory for clinical trials, congratulates client company Shasqi on the announcement of the first-ever application of click chemistry in humans, with the launch of the Company’s lead clinical candidate, SQ3370. Shasqi is the first Y Combinator-backed biotech company to reach clinical development.
Author By Agilex BiolabsPosted on
Title: Development and Validation of a PK Method for Tocilizumab in Both Singlicate and Duplicate on the Gyrolab Platform
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s most advanced specialist bioanalytical laboratory for clinical trials, congratulated client Zelira Therapeutics on their Phase 2 Medicinal Cannabis Insomnia Study.
Author By Agilex BiolabsPosted on
Why Australia is the World’s Leading Early Phase Destination – Rapid Start-up, No IND Required, and a Government-Backed Refund on Almost Half of all Trial Costs Increasingly Sponsors are wanting to be actively involved in the core clinical partner selection because they matter to the success of their clinical research.
Author By Agilex BiolabsPosted on
Hi, everyone. I'm Arsalan Arif, the publisher of Endpoints News, and I'm pleased to be your moderator today. Today's webinar is sponsored by Agilex Biolabs in Australia. Our topic is Why Australia is the World's Leading Early Phase Destination - Rapid Start-up, No IND Required, and a Government-Backed Refund on Almost Half of all Trial Costs. I'm joined by two great guests today. Kurt Sales, the Director of Immunoassay at Agilex Biolabs, and Jane Kelly, the CEO of CMAX Clinical Research.
Author By Agilex BiolabsPosted on
Award-winning Agilex Biolabs, the Asia-Pacific region’s leading bioanalytical laboratory for clinical trials, based in Australia, has developed the world’s most accurate cannabinoid assay.
Author By Agilex BiolabsPosted on
Gathering together the key clinical service providers for a trial can be a daunting process – especially if you are going off-shore to reactivate trials or start new trials to make up for COVID delays.
However, Australia offers a refreshingly streamlined process because of its group of proven top-tier providers that regularly work together so are fully engaged and in sync with processes and each other’s requirements and capabilities. This makes setting up trials in Australia a smooth, professional, and rapid process for biotechs. This webinar looks at how moving trials to Australia could be much easier than you think.
However, Australia offers a refreshingly streamlined process because of its group of proven top-tier providers that regularly work together so are fully engaged and in sync with processes and each other’s requirements and capabilities. This makes setting up trials in Australia a smooth, professional, and rapid process for biotechs. This webinar looks at how moving trials to Australia could be much easier than you think.
Author By Agilex BiolabsPosted on
All right. Hi everybody. I’m Arsalan Arif, the publisher of Endpoints News and I’m pleased to be your moderator today. Today’s webinar is sponsored by Agilex Biolabs in Australia and our topic is the Australian Advantage, de-risk your early phase trials. I’m joined by Dr Kurt Sales today with Agilex Biolabs. He’s got an approximately 20 minute presentation and we’re going to follow it up with a quick Q&A. And now I give you Dr Kurt Sales.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest specialist bioanalytical laboratory for clinical trials, and Endpoints News launched a new webinar “How Easy is it to Move Your Trials to Australia” at BIO Asia-Taiwan 2020.
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s largest specialist bioanalytical laboratory for biotech clinical trials, is presenting the Australian Advantage including the world’s most attractive rebate on clinical trials costs, at BIO Korea 2020.
Author By Agilex BiolabsPosted on
The reality is, Australia has fantastic key opinion leaders, some great principal investigators that have been running clinical trials for decades. The CROs, RFA with FDA and EMA requirements. And in fact, our whole Australian service provision industry sets itself to that standard because ultimately that is the client base that Australia is servicing. Most CROs in Australia and service providers will be able to talk about situations where their data has been presented to the FDA or EMA with success.
Author By Agilex BiolabsPosted on
Video https://www.youtube.com/watch?v=sCUT8qtlRX4&feature=youtu.be...
Author By Agilex BiolabsPosted on
Award-winning Australian bioanalytical laboratory Agilex Biolabs announced today it has appointed US-based Dr Caroline Popper, MBBS, MPH as Board Chair as part of its APAC clinical trials bioanalytics growth plans.
Author By Agilex BiolabsPosted on
Award-winning Australian bioanalytical laboratory Agilex Biolabs announced today it has appointed PhD and NHMRC Fellow Patrick Hughes as R&D Manager Immunobiology.
Author By Agilex BiolabsPosted on
Award-winning Agilex Biolabs, the Asia-Pacific region’s leading bioanalytical laboratory based in Australia, announced a raft of new pharmacodynamics services for biotech and pharma clients conducting preclinical and clinical trials in Asia, US, EU and Australia.
Author By Agilex BiolabsPosted on
Australia-based Agilex Biolabs announced a broad range of new pharmacodynamics services for biopharma clients running preclinical and clinical trials in the U.S., Asia, European Union and Australia.
Author By Agilex BiolabsPosted on
Award-winning Agilex Biolabs, the Asia-Pacific region’s leading bioanalytical laboratory based in Australia, announced a raft of new pharmacodynamics services for biotech and pharma clients conducting preclinical and clinical trials in Asia, US, EU and Australia.
Author By Agilex BiolabsPosted on
One of the most important drug development start-up decisions is finding a centrally located bioanalytical laboratory that can support clinical research across all the study locations, and through pre-clinical then potentially for all Phases.
Quality control and consistency of sample management and analysis from early Phase onwards can make a real difference to quality of data and final outcomes.
Quality control and consistency of sample management and analysis from early Phase onwards can make a real difference to quality of data and final outcomes.
Author By Agilex BiolabsPosted on
Australia-based Agilex Biolabs, the Asia-Pacific region’s leading bioanalytical laboratory for global clients conducting preclinical and clinical trials, announced at AusBiotech 2019 today it has been selected as a Top 10 Bioanalytical Services Provider by PharmaTech Outlook Magazine (Booth #523).
Author By Agilex BiolabsPosted on
Boston, MA – Agilex Biolabs, the Asia Pacific’s leading bioanalytical laboratory, announced...
Author By Agilex BiolabsPosted on
https://youtu.be/UrlJI-Y--k0...
Author By Agilex BiolabsPosted on
Agilex Biolabs, Australia’s leading bioanalytical laboratory for global clients
conducting preclinical and clinical trials announced at DIA 2019 this week it will analyse more than 60,000 samples in 2019 (June 23-27 San Diego, CA) Booth 2006.
conducting preclinical and clinical trials announced at DIA 2019 this week it will analyse more than 60,000 samples in 2019 (June 23-27 San Diego, CA) Booth 2006.